Details of the Drug
General Information of Drug (ID: DMJ0QOW)
| Drug Name |
Rilpivirine
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
500287-72-9; TMC278; Edurant; TMC 278; UNII-FI96A8X663; R278474; TMC-278; 4-{[4-({4-[(E)-2-Cyanoethenyl]-2,6-Dimethylphenyl}amino)pyrimidin-2-Yl]amino}benzonitrile; CHEBI:68606; FI96A8X663; R 278474; 4-{[4-({4-[(E)-2-cyanovinyl]-2,6-dimethylphenyl}amino)pyrimidin-2-yl]amino}benzonitrile; (E)-4-((4-((4-(2-cyanovinyl)-2,6-dimethylphenyl)amino)pyrimidin-2-yl)amino)benzonitrile; (E)-4-(4-(4-(2-cyanovinyl)-2,6-dimethylphenylamino)pyrimidin-2-ylamino)benzonitrile; W-202888; RPV; Mu O-conotoxin
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Human Immunodeficiency VirusHumans and other mammals
|
||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
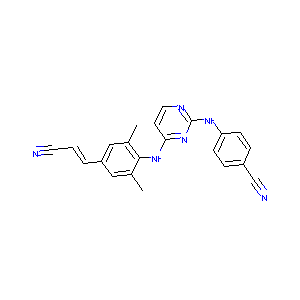 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 366.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.5 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Human immunodeficiency virus infection | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 1C62 | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Rilpivirine
Coadministration of a Drug Treating the Disease Different from Rilpivirine (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | 2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4. | ||||
|---|---|---|---|---|---|
| 2 | Structures of muO-conotoxins from Conus marmoreus. I nhibitors of tetrodotoxin (TTX)-sensitive and TTX-resistant sodium channels in mammalian senso... J Biol Chem. 2004 Jun 11;279(24):25774-82. | ||||
| 3 | Australian Government: Australian Public Assessment Report for Rilpivirine | ||||
| 4 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 5 | FDA Approved Drug Products: Edurant (rilpivirine) oral tablets | ||||
| 6 | Safety, efficacy, and pharmacokinetics of rilpivirine: systematic review with an emphasis on resource-limited settings. HIV AIDS (Auckl). 2011;3:35-44. doi: 10.2147/HIV.S14559. Epub 2011 Apr 28. | ||||
| 7 | Duffey H, Firszt R: Management of acute attacks of hereditary angioedema: role of ecallantide. J Blood Med. 2015 Apr 16;6:115-23. doi: 10.2147/JBM.S66825. eCollection 2015. | ||||
| 8 | Emerging antiviral drugs. Expert Opin Emerg Drugs. 2008 Sep;13(3):393-416. | ||||
| 9 | Potential of the novel antiretroviral drug rilpivirine to modulate the expression and function of drug transporters and drug-metabolising enzymes in vitro. Int J Antimicrob Agents. 2013 May;41(5):484-7. | ||||
| 10 | Human biotransformation of the nonnucleoside reverse transcriptase inhibitor rilpivirine and a cross-species metabolism comparison. Antimicrob Agents Chemother. 2013 Oct;57(10):5067-79. | ||||
| 11 | Population pharmacokinetics and pharmacogenetics analysis of rilpivirine in HIV-1-infected individuals. Antimicrob Agents Chemother. 2016 Dec 27;61(1). | ||||
| 12 | Product Information. Rukobia (fostemsavir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 13 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 14 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 15 | Product Information. Edurant (rilpivirine). Tibotec Pharmaceuticals, Titusville, NJ. | ||||
| 16 | Product Information. Xospata (gilteritinib). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 17 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 18 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 19 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 20 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 21 | Canadian Pharmacists Association. | ||||
| 22 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 23 | Bengtsson B, Fagerstrom PO "Extrapulmonary effects of terbutaline during prolonged administration." Clin Pharmacol Ther 31 (1982): 726-32. [PMID: 7042176] | ||||
| 24 | Cerner Multum, Inc. "Canadian Product Information.". | ||||
| 25 | Product Information. Austedo (deutetrabenazine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 26 | Product Information. Victrelis (boceprevir). Schering-Plough Corporation, Kenilworth, NJ. | ||||
| 27 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 28 | Product Information. Xalkori (crizotinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 29 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 30 | Product Information. Tagrisso (osimertinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 31 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 32 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 33 | Product Information. Braftovi (encorafenib). Array BioPharma Inc., Boulder, CO. | ||||
| 34 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 35 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 36 | Product Information. Macrilen (macimorelin). Aeterna Zentaris, Charleston, SC. | ||||
| 37 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 38 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
